Both sodium chlorite and hydrogen peroxide are powerful oxidizing agents widely used for disinfection, bleaching, and oxidation across industrial, environmental, and healthcare sectors. While both serve similar functions, their chemical properties, mechanisms of action, stability, and environmental impacts differ significantly. Understanding these differences is crucial to selecting the right oxidizer for your specific process or treatment application.
What Is Sodium Chlorite?
Sodium chlorite (NaClO₂) is a chlorine-based oxidizer that produces chlorine dioxide (ClO₂) when activated by an acid or oxidizing agent. Chlorine dioxide is known for its strong and selective disinfection performance, making sodium chlorite a key compound in water treatment and industrial sanitation.
Key Properties
- Chemical Formula: NaClO₂
- Oxidizing Strength: Strong; generates chlorine dioxide gas when activated
- Appearance: White crystalline solid, soluble in water
- pH: Typically alkaline
- Storage Stability: More stable than hydrogen peroxide under neutral or basic conditions
Advantages
Generates chlorine dioxide, which is powerful yet less corrosive than chlorine.
Performs effectively across a wide pH range and remains active even in the presence of organic matter.
Excellent for biofilm removal and microbial disinfection in water systems.
Common Applications
Water and wastewater treatment
Food and beverage sanitation
Pulp and paper bleaching
Odor and mold control
What Is Hydrogen Peroxide?
Hydrogen peroxide (H₂O₂) is an oxygen-based oxidizer valued for its eco-friendly and residue-free properties. When decomposed, it releases water and oxygen, making it an environmentally sustainable disinfectant and bleaching agent.
Key Properties
- Chemical Formula: H₂O₂
- Oxidizing Strength: Strong but non-selective
- Appearance: Clear, colorless liquid
- Decomposition Products: Water and oxygen
- Stability: Sensitive to light, heat, and contaminants; decomposes easily under stress
Advantages
Environmentally safe - leaves no harmful byproducts.
Effective for surface sterilization, oxidation of organics, and bleaching.
Commonly used in medical, industrial, and environmental applications.
Common Applications
Medical sterilization and wound cleaning
Textile and industrial bleaching
Environmental remediation
Food and beverage disinfection
Key Differences Between Sodium Chlorite and Hydrogen Peroxide
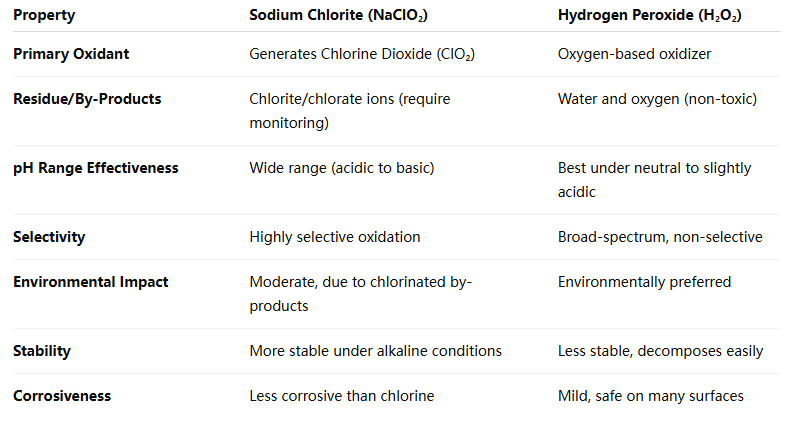
Which Should You Use?
Choose Sodium Chlorite when: You need powerful and selective oxidation - ideal for water treatment, biofilm control, or industrial disinfection where chlorine dioxide’s effectiveness is beneficial.
Choose Hydrogen Peroxide when: Environmental safety and low residue are top priorities, such as in medical sterilization, textile bleaching, or household cleaning applications.
Additional Considerations
Water Treatment: Hydrogen peroxide is effective for oxidizing contaminants like iron and sulfur and acts faster than chlorine. Sodium chlorite is primarily used to generate chlorine dioxide for pathogen control and odor management.
Surface Cleaning: Hydrogen peroxide is gentler on fabrics, plastics, and coatings, while sodium chlorite (and its derived ClO₂) can be more aggressive on delicate materials.
Biofilm Removal: Both compounds outperform many common disinfectants, including quaternary ammonium compounds, in biofilm elimination.
Safety Note
Both sodium chlorite and hydrogen peroxide are strong oxidizers and must be handled with care:
Wear protective gloves, goggles, and clothing.
Avoid mixing with combustible materials.
Store in well-ventilated areas away from direct sunlight.
Follow manufacturer and regulatory guidelines for safe handling and disposal.
Conclusion
When comparing Sodium Chlorite vs Hydrogen Peroxide, the best choice depends on your application. Sodium chlorite excels in industrial and water disinfection, where strong oxidation and chlorine dioxide generation are required. Hydrogen peroxide, on the other hand, is ideal for eco-friendly sterilization and bleaching, offering a safer and residue-free alternative.
By understanding their differences in chemistry, stability, and performance, you can select the most effective oxidizer to enhance safety, efficiency, and environmental compliance in your process.



